 Definition:LDF are a type of force acting between atoms and molecules.
Definition:LDF are a type of force acting between atoms and molecules.
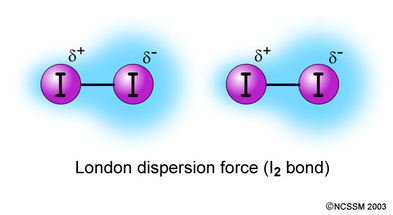
London Dispersion Force Definition:London dispersion force is a weak intermolecular force between two atomsor molecules in close proximity of each other. The force is a quantum force generated byelectron repulsion between the electron clouds of two atoms or molecules as they approach each other.
The London dispersion force is the weakest of the van der Waals forces and is the force that causesnonpolar atoms or molecules to condense into liquids or solids as temperature is lowered.
Also Known As: London forces, LDF, dispersion forces, instantaneous dipole forces, induced dipole forces.
No comments:
Post a Comment